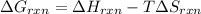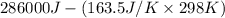Chemistry, 25.09.2019 02:20 destinyhammons12345
Determine the electrical work required to produce one mole of hydrogen in the electrolysis of liquid water at 298°k and 1 atm. the chemical reaction is h2001) h2(g) + 0.502(g) data (at 298°k and 1 atm): ah = 286 kj for this reaction, suzo = 70 jk, sh2 = 131 jik, and soz = 205 j/ºk.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What mass of carbon dioxide is produced from the complete combustion of 4.50×10−3 g of methane?
Answers: 2

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 18:00
Answer asap need it by wednesday morning carry out the following calculations on ph and ka of from data. i. calculate the ph of 0.02m hcl ii. calculate the ph of 0.036m naoh iii. calculate the ph of 0.36m ca(oh)2 iv. calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 v. calculate ka for weak acid ha which has a ph of 3.65 at 0.30m concentration vi. calculate the ka of a solution made by mixing 15.0 cm3 0.2m ha and 60.0 cm3 0.31m a-. [ph= 3.80] vii. calculate the ph of a solution made by mixing 15.0 cm3 0.1m naoh and 35.0 cm3 0.2m hcooh. [ka = 1.82 x 10-4 m]
Answers: 1

Chemistry, 22.06.2019 19:00
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1
You know the right answer?
Determine the electrical work required to produce one mole of hydrogen in the electrolysis of liquid...
Questions



Mathematics, 15.09.2021 07:00

Mathematics, 15.09.2021 07:00

English, 15.09.2021 07:00





English, 15.09.2021 07:00


History, 15.09.2021 07:00

Mathematics, 15.09.2021 07:00


Chemistry, 15.09.2021 07:00



History, 15.09.2021 07:00

Mathematics, 15.09.2021 07:00

Mathematics, 15.09.2021 07:00

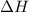 = 286 kJ =
= 286 kJ = 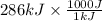
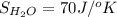 ,
, 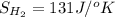
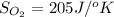
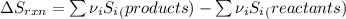
![[(\frac{1}{2} \times S_{O_{2}}) - (1 \times S_{H_{2}})] - [1 \times S_{H_{2}O}]](/tpl/images/0260/0439/5a90c.png)
![[(\frac{1}{2} \times 205) + (1 \times 131)] - [(1 \times 70)]](/tpl/images/0260/0439/31e9d.png)