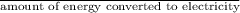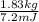Calculate the amount of co2 (in kg) released when 1 kg coal is burnt. assume that carbon content of the coal is 50% by mass. 4. calculate the co2 production in kg/mj if a coal fired power plant (efficiency - 30%) is used to produce electricity. assume energy density of coal - 24 mj/kg and assume the coal is

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Achef makes salad dressing by mixing oil, vinegar, and spices, as shown. which type of matter is the salad dressing?
Answers: 1

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 15:30
Why does earth rotate? because earth is formed from cold gases collapsing due to gravity because the matter in the nebula that formed earth was spinning because earth forms more than 99% of the mass of the solar system because the hydrogen atoms inside the nebula fused to form helium
Answers: 1
You know the right answer?
Calculate the amount of co2 (in kg) released when 1 kg coal is burnt. assume that carbon content of...
Questions

Computers and Technology, 04.08.2019 20:30






Biology, 04.08.2019 20:30

Mathematics, 04.08.2019 20:30


Physics, 04.08.2019 20:30


Mathematics, 04.08.2019 20:30

Biology, 04.08.2019 20:30

Mathematics, 04.08.2019 20:30

Biology, 04.08.2019 20:30


Mathematics, 04.08.2019 20:30

History, 04.08.2019 20:30

History, 04.08.2019 20:30

English, 04.08.2019 20:30

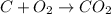
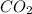 = 44 kg/kmol
= 44 kg/kmol
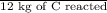
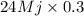 = 7.2 Mj
= 7.2 Mj