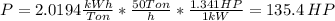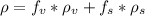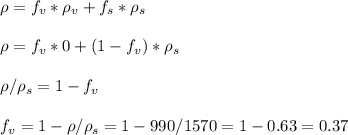You are requested to reduce the size of 50 ton/hr of a given solid. the size of the feed is such 80% passes a 4-in (76.2 mm) screen and 80 % of the product passes a (1/4) inch ( 3.175 mm). knowing the work index of the solid (ei=9.45), calculate the required power for the operation in hp. activity ii a packed bed is composed of spheres having a diameter of 12 mm. the bulk density of the overall packed bed is 990 kg/m3 and the density of solid spheres is 1570 kg/m3. calculate the void fraction of the bed.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2

Chemistry, 22.06.2019 11:00
Predict the products of the following acid-base reactions, and predict whether the equilibrium lies to the left or to the right of the reaction arrow.part ao2-(aq)+h2o(l)< => express your answer as part of a chemical equation. identify all of the phases in your answer.o2-(aq)+h2o(l) < => oh-(aq)+oh-(aq)part bpredict whether the equilibrium lies to the left or to the right of the equation in previous part.h2o is a stronger acid than oh–, so the equilibrium lies to the right.h2o is a weaker acid than oh–, so the equilibrium lies to the left.h2o is a stronger acid than oh–, so the equilibrium lies to the left.h2o is a weaker acid than oh–, so the equilibrium lies to the right.part cch3cooh(aq)+hs? (aq) < => express your answer as part of a chemical equation. identify all of the phases in your answer.ch3cooh(aq)+hs-(aq) < => h2s(aq)+c2h3o2-(aq)h2s(aq)+c2h3o2-(aq)part dpredict whether the equilibrium lies to the left or to the right of the equation in previous part.ch3cooh is a weaker acid than h2s, so the equilibrium lies to the right.ch3cooh is a weaker acid than h2s, so the equilibrium lies to the left.ch3cooh is a stronger acid than h2s, so the equilibrium lies to the right.ch3cooh is a stronger acid than h2s, so the equilibrium lies to the left.part eno2-(aq)+h2o(l) < => express your answer as part of a chemical equation. identify all of the phases in your answer.no2-(aq)+h2o(l) < => part fpredict whether the equilibrium lies to the left or to the right of the equation in previous part.hno2 is a stronger acid than h2o, so the equilibrium lies to the right.hno2 is a weaker acid than h2o, so the equilibrium lies to the left.hno2 is a stronger acid than h2o, so the equilibrium lies to the left.hno2 is a weaker acid than h2o, so the equilibrium lies to the right.
Answers: 1

Chemistry, 22.06.2019 21:30
How can the periodic table be used to predict the behavior of elements?
Answers: 1

You know the right answer?
You are requested to reduce the size of 50 ton/hr of a given solid. the size of the feed is such 80%...
Questions

Mathematics, 09.12.2019 08:31

Mathematics, 09.12.2019 08:31

Mathematics, 09.12.2019 08:31



Biology, 09.12.2019 08:31




English, 09.12.2019 08:31

Mathematics, 09.12.2019 08:31



Business, 09.12.2019 08:31


Mathematics, 09.12.2019 08:31

Mathematics, 09.12.2019 08:31

Mathematics, 09.12.2019 08:31


History, 09.12.2019 08:31