Chemistry, 25.09.2019 02:00 AriesDaWolf
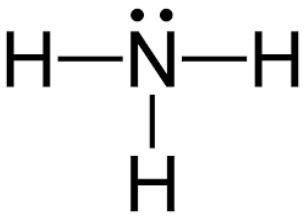
Which of the following explains the vsepr geometry of an ammonia molecule?
it is tetrahedral because there are four bonded pairs around nitrogen.
it is trigonal pyramidal because there are four bonded pairs around nitrogen.
it is tetrahedral because there are three bonded pairs and one lone pair around nitrogen.
it is trigonal pyramidal because there are three bonded pairs and one lone pair around nitrogen.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3

Chemistry, 23.06.2019 03:00
Give a real-world example of an energy transformation that uses two of the following forms of energy: chemical, mechanical, nuclear, gravitational, radiant, electrical, thermal (heat), and/or sound.
Answers: 3
You know the right answer?
Which of the following explains the vsepr geometry of an ammonia molecule?
it is tetrahedral...
it is tetrahedral...
Questions

Computers and Technology, 17.09.2021 14:00

Physics, 17.09.2021 14:00


English, 17.09.2021 14:00





Social Studies, 17.09.2021 14:00

Biology, 17.09.2021 14:00


English, 17.09.2021 14:00



Social Studies, 17.09.2021 14:00

History, 17.09.2021 14:00


Mathematics, 17.09.2021 14:00


Social Studies, 17.09.2021 14:00