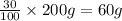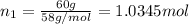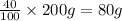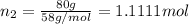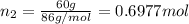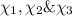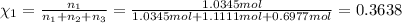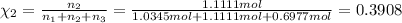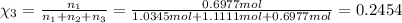Chemistry, 25.09.2019 02:00 jaylabazemore
Two hundred kg of liquid contains 30% butane, 40% pentane, and the rest hexane (mass %) determine: the mole fraction composition of the liquid the mass fraction composition on hexane free basis 1. 2.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2

Chemistry, 23.06.2019 01:00
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1

You know the right answer?
Two hundred kg of liquid contains 30% butane, 40% pentane, and the rest hexane (mass %) determine:...
Questions

Health, 27.05.2021 04:30

Mathematics, 27.05.2021 04:30

Mathematics, 27.05.2021 04:30





Mathematics, 27.05.2021 04:30

English, 27.05.2021 04:30

English, 27.05.2021 04:30

Mathematics, 27.05.2021 04:30

Biology, 27.05.2021 04:30

Mathematics, 27.05.2021 04:30

Mathematics, 27.05.2021 04:30





Health, 27.05.2021 04:30

Mathematics, 27.05.2021 04:30