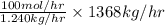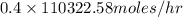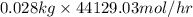Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
0.66y = 0.9x + 0.48 if y has a value of 108.45 what is the value of x?
Answers: 1

Chemistry, 21.06.2019 22:30
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution? a. 3.88 m, b. 1.03 m, c. 1.5 m, d. 15.5 m
Answers: 3

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 22.06.2019 22:30
[ou.03jthe pictures below show the wavelengths and intensities of electromagnetic radiations emitted by three stars, star 1, star 2, and star 3. intensity intensity- intensity- 1000 3500 6000 8500 11000 wavelength (a) star 1 1000 3500 6000 8500 11000 1000 3500 6000 8500 11000 wavelength (a) wavelength (a) star 2 star 3 which of these statements is correct about the color of the three stars? star 2 is white in color o star 2 is yellow in color star 1 and star 3 are yellow in color star 1 and star 3 are white in color
Answers: 1
You know the right answer?
Pure nitrogen (n2) and pure hydrogen (h2) are fed to a mixer. the product stream has 40.0% mole nitr...
Questions

English, 21.10.2020 02:01


Mathematics, 21.10.2020 02:01


Chemistry, 21.10.2020 02:01

Chemistry, 21.10.2020 02:01

Chemistry, 21.10.2020 02:01

Mathematics, 21.10.2020 02:01


Biology, 21.10.2020 02:01

Mathematics, 21.10.2020 02:01




History, 21.10.2020 02:01




Business, 21.10.2020 02:01

Mathematics, 21.10.2020 02:01

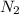 in feed = 40 mole%
in feed = 40 mole%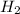 in feed = (100 - 40)% = 60%
in feed = (100 - 40)% = 60%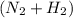 in feed stream.
in feed stream.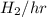 (2 g/mol of
(2 g/mol of 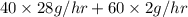
 (as 1 kg = 1000 g)
(as 1 kg = 1000 g)