
Chemistry, 25.09.2019 01:20 eatheng4441
Ch4 with pressure 1 atm and volume 10 liter at 27°c is passed into a reactor with 20% excess oxygen, how many moles of oxygen is left in the products?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:40
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 23.06.2019 01:00
An unsaturated hydrocarbon is a hydrogen-carbon compound with a. a network solid structure b. single bonds c. single bonds in a branched-chain structure d. double or triple bonds
Answers: 1
You know the right answer?
Ch4 with pressure 1 atm and volume 10 liter at 27°c is passed into a reactor with 20% excess oxygen,...
Questions


Biology, 11.10.2021 21:20


World Languages, 11.10.2021 21:20

Mathematics, 11.10.2021 21:20

Computers and Technology, 11.10.2021 21:20



Social Studies, 11.10.2021 21:20

Chemistry, 11.10.2021 21:20





Mathematics, 11.10.2021 21:30

Biology, 11.10.2021 21:30




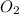 left in the products are 0.16 moles.
left in the products are 0.16 moles.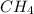 .
.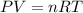
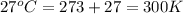
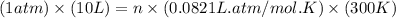
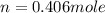
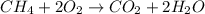
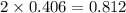 moles of
moles of 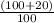 × Required moles of
× Required moles of 

