
Agas mixture of 20 moles nitrogen and 70 moles hydrogen is fed to a reactor in which ammonia is produced. assuming that the reaction proceeds to completion, determine the composition of the exit gas stream both in mole and mass %. if 75% of nitrogen gets converted, what will be the corresponding fractions?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3
You know the right answer?
Agas mixture of 20 moles nitrogen and 70 moles hydrogen is fed to a reactor in which ammonia is prod...
Questions












English, 21.01.2020 18:31


Mathematics, 21.01.2020 18:31

Law, 21.01.2020 18:31


English, 21.01.2020 18:31

Biology, 21.01.2020 18:31

Mathematics, 21.01.2020 18:31

Mathematics, 21.01.2020 18:31

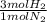 = 60 moles of H₂(g)
= 60 moles of H₂(g) 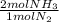 = 40 moles of NH₃(g)
= 40 moles of NH₃(g)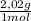 = 20,2 g of H₂
= 20,2 g of H₂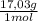 = 681,2 g of NH₃
= 681,2 g of NH₃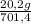 ₓ 100 = 2,9% H₂;
ₓ 100 = 2,9% H₂; 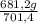 ₓ100 = 97,1% NH₃
ₓ100 = 97,1% NH₃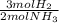 = 45 moles of H₂(g)
= 45 moles of H₂(g) 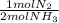 = 15 moles of N₂(g)
= 15 moles of N₂(g) 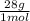 = 140 g of N₂
= 140 g of N₂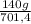 ₓ 100 =20% N₂;
ₓ 100 =20% N₂; 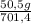 ₓ 100 = 7,2% H₂;
ₓ 100 = 7,2% H₂; 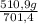 ₓ100 = 72,8% NH₃
ₓ100 = 72,8% NH₃

