Chemistry, 25.09.2019 01:00 vinp190p9zekn
One mole of pure n2 and 1 mole of pure o2 are contained in separate compartments of a rigid and insulated container at 1 bar and 298 k. the separator between the compartments are later removed to allow the mixing of the gases. assume that both gases are in the ideal gas state.
a) what is the final t and p of the mixture?
b) for a mixture of ideal gases, each component carries a partial pressure that is proportional to its mole fraction: i. e., = p and = p, p being the total pressure of the mixture. calculate the entropy change of the mixing process and draw a schematic showing the hypothetical path you used for the calculation.
i am unclear why you need enthalpy information when the question asks for entropy. can someone solve part a?

Answers: 3


Another question on Chemistry



Chemistry, 23.06.2019 09:00
What properties would have caused early researchers to name hydrogen "inflammable air”
Answers: 3

Chemistry, 23.06.2019 10:30
Identify the limiting reactant when 9.65-g h2so4 reacts with 6.10-g of naoh.the equation is h2s04 + 2naoh = 2h2o + na2so4• what is the theoretical yield of na2so4, in grams? • how much of the excess reagent will remain after the reaction has been completed? • if 10.5-g of na2so4 are actually recovered experimentally, what is the percent yield?
Answers: 3
You know the right answer?
One mole of pure n2 and 1 mole of pure o2 are contained in separate compartments of a rigid and insu...
Questions

English, 16.04.2020 03:54

Mathematics, 16.04.2020 03:54

Physics, 16.04.2020 03:54



Mathematics, 16.04.2020 03:54


Social Studies, 16.04.2020 03:54




History, 16.04.2020 03:54

Mathematics, 16.04.2020 03:54







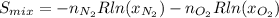
![S_{mix}=-(1mol)[(8.314J/(mol*K)]ln(0.5)-(1mol)[(8.314J/(mol*K)]ln(0.5)](/tpl/images/0259/8106/ed779.png)