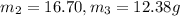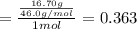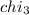Chemistry, 25.09.2019 00:20 latdoz0952
Aliquid mixture contains water (h2o, mw = 18.0), ethanol (c2h5oh, mw = 46.0) and methanol (ch3oh, mw = 32.0). using two different analytical techniques to analyze the mixture, it was determined that the water mole fraction was 0.250 while the water mass fraction was 0.134. determine the mole fraction ethanol (c2h5oh) and the mole fraction methanol (ch3oh) in the solution. report the values to the correct number of significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 21:00
Need what is special about water as a compound? how does water regulate climate? what drives water evaporation? why is the water vapor fresh water when it rises from the ocean? why might freshwater in the form of snow take longer to enter the water cycle again than liquid precipitation? what is an aquifer? what role do people play in the water cycle? plz just answer as many as you can ! thx if you !
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1
You know the right answer?
Aliquid mixture contains water (h2o, mw = 18.0), ethanol (c2h5oh, mw = 46.0) and methanol (ch3oh, mw...
Questions















Biology, 05.03.2020 17:36




Mathematics, 05.03.2020 17:37

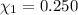
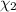
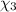
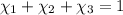
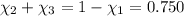

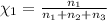
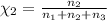
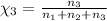
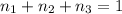
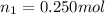
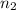
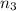

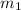
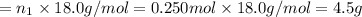

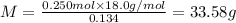
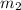
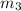
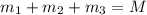
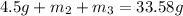
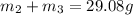 ..[1]
..[1]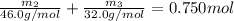
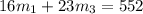 ..[2]
..[2]