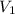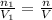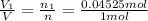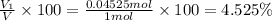Chemistry, 24.09.2019 23:30 zanaplen27
Deep sea divers use a mixture of helium and oxygen to breathe. assume that a diver is going to a depth of 150 feet where the total pressure is 4.42 atm. the partial pressure of oxygen at this depth is to be maintained at 0.20 atm, the same as at sea level. what must be the percent by volume of oxygen in the gas mixture?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:40
Darla claims that the first periodic table developed by mendeleev was not completely accurate, so it is not useful at all. harmony argues that it establish the periodic table we use today, making it more credible. who is correct and why? darla is correct, because a model that has any mistakes should be thrown out. darla is correct, because a good model would not need to change. harmony is correct, because mendeleev’s model had all of the information correct in the first version. harmony is correct, because mendeleev’s model made predictions that came true.
Answers: 1


Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 22:00
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2
You know the right answer?
Deep sea divers use a mixture of helium and oxygen to breathe. assume that a diver is going to a dep...
Questions

English, 01.10.2019 00:30

Mathematics, 01.10.2019 00:30

Health, 01.10.2019 00:30

English, 01.10.2019 00:30


Health, 01.10.2019 00:30

Mathematics, 01.10.2019 00:30


Mathematics, 01.10.2019 00:30

Mathematics, 01.10.2019 00:30


Social Studies, 01.10.2019 00:30


Health, 01.10.2019 00:30


Mathematics, 01.10.2019 00:30

Mathematics, 01.10.2019 00:30

Mathematics, 01.10.2019 00:30


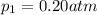
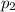
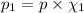 (Dalton law of partial pressure)
(Dalton law of partial pressure)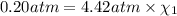
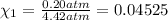
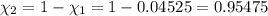
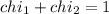
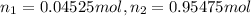
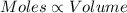 (At temperature and pressure)
(At temperature and pressure)