
Chemistry, 24.09.2019 20:30 melidacampos12
Assume that the van der waals b constant for xenon (see table 1c.3- or 1.6 old version - van der waals coefficients) divided by avogadro's number represents the volume of a single xenon atom. assume that xenon atom is spherical, and estimate the radius (in meters) of a xenon atom using the formula vsphere = (4/3)rır3.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1
You know the right answer?
Assume that the van der waals b constant for xenon (see table 1c.3- or 1.6 old version - van der waa...
Questions

Mathematics, 07.07.2019 16:00

Mathematics, 07.07.2019 16:00


Mathematics, 07.07.2019 16:00

Mathematics, 07.07.2019 16:00


Mathematics, 07.07.2019 16:00

Health, 07.07.2019 16:00

Chemistry, 07.07.2019 16:00

Mathematics, 07.07.2019 16:00

Health, 07.07.2019 16:00

English, 07.07.2019 16:00

Mathematics, 07.07.2019 16:00


History, 07.07.2019 16:00


Mathematics, 07.07.2019 16:00

Mathematics, 07.07.2019 16:00

Mathematics, 07.07.2019 16:00

Mathematics, 07.07.2019 16:00

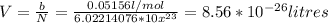
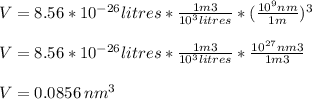

![r=\sqrt[3]{\frac{3V}{4\pi} }= \sqrt[3]{\frac{3*0.0856nm^{3}}{4\pi} }=\sqrt[3]{0.0204 nm^{3} }=0.2734nm](/tpl/images/0259/1334/d0347.png)


