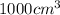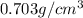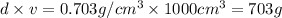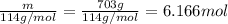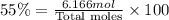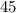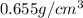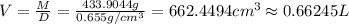Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2

Chemistry, 22.06.2019 22:30
Which of these statements best explains why space exploration should be encouraged? it prepares humans to live without oxygen. it dispel myths about objects in space. it prevents comets and asteroids from striking earth. it creates technology to absorb harmful radiations in space.
Answers: 1
You know the right answer?
Hexane and octane are mixed to form a 45 mol% hexane solution at 25 deg c. the densities of hexane a...
Questions

Mathematics, 07.01.2021 14:00

Mathematics, 07.01.2021 14:00

Health, 07.01.2021 14:00

Mathematics, 07.01.2021 14:00


English, 07.01.2021 14:00

Mathematics, 07.01.2021 14:00

Chemistry, 07.01.2021 14:00

Social Studies, 07.01.2021 14:00

English, 07.01.2021 14:00

Arts, 07.01.2021 14:00



English, 07.01.2021 14:00

Mathematics, 07.01.2021 14:00

Social Studies, 07.01.2021 14:00

Mathematics, 07.01.2021 14:00

Physics, 07.01.2021 14:00

English, 07.01.2021 14:00

Advanced Placement (AP), 07.01.2021 14:00