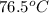Chemistry, 24.09.2019 20:00 andrecoral105
Amixture of 454 kg of applesauce at 10 degrees celsius is heated in a heat exchanger by adding 121300 kj. calculate the outlet temperature of the applesauce. (hint: heat capacity for applesauce is given at 32.8 degrees celsius. assume that this is constant and use this as the average.)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3


Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1

Chemistry, 23.06.2019 00:40
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1
You know the right answer?
Amixture of 454 kg of applesauce at 10 degrees celsius is heated in a heat exchanger by adding 12130...
Questions


Health, 08.12.2021 22:40

Geography, 08.12.2021 22:40


SAT, 08.12.2021 22:40


English, 08.12.2021 22:40


Mathematics, 08.12.2021 22:40


History, 08.12.2021 22:40

Geography, 08.12.2021 22:40




Chemistry, 08.12.2021 22:40

SAT, 08.12.2021 22:40


Mathematics, 08.12.2021 22:40

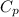 ) of apple sauce at
) of apple sauce at 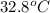 = 4.0177
= 4.0177 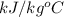
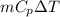
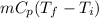
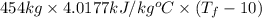
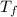 =
=