Chemistry, 24.09.2019 20:00 prettygirllniyiaa
The active ingredient in aspirin is acetylsalicylic acid. in a lab class, a student uses paper chromatography to isolate another common ingredient of headache remedies. the sample of this ingredient had a mass of 384 mg and a volume of 0.32 cm3. looking at the following data, what was the other ingredient in the headache remedy? white table sugar caffeine sodium chloride d -0.70 g/ml d 1.2 g/ml d 2.2 g/ml

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 07:20
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1
You know the right answer?
The active ingredient in aspirin is acetylsalicylic acid. in a lab class, a student uses paper chrom...
Questions


Social Studies, 10.10.2019 15:10

History, 10.10.2019 15:10


Mathematics, 10.10.2019 15:10

Biology, 10.10.2019 15:10

Mathematics, 10.10.2019 15:10

Health, 10.10.2019 15:10

History, 10.10.2019 15:10




Chemistry, 10.10.2019 15:10

Mathematics, 10.10.2019 15:10



Mathematics, 10.10.2019 15:10

Mathematics, 10.10.2019 15:10

History, 10.10.2019 15:10

Mathematics, 10.10.2019 15:10

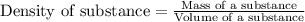
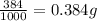 (1g=1000mg)
(1g=1000mg)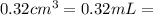 (Conversion factor:
(Conversion factor: 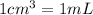 )
)