
Chemistry, 24.09.2019 20:00 leelee8335
You notice that the water in your friend's swimming pool is cloudy and that the pool walls are discolored at the water line. a quick analysis reveals that the ph of the water is 8.00 when it should be 7.20. the pool is 9.00 m wide, 15.0 m long, and has an average depth of 2.50m. what is the minimum (in the absence of any buffering capacity) volume (ml) of 12.0 wt% h2so4 (sg=1.080) that should be added to return the pool to the desired ph? in ml

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1


Chemistry, 23.06.2019 00:30
Balance the following reaction. as2s3 + 9o2 → 2as2o3 + so2
Answers: 2
You know the right answer?
You notice that the water in your friend's swimming pool is cloudy and that the pool walls are disco...
Questions

Chemistry, 25.03.2021 01:00

English, 25.03.2021 01:00

History, 25.03.2021 01:00


Mathematics, 25.03.2021 01:00




Mathematics, 25.03.2021 01:00




Mathematics, 25.03.2021 01:00





English, 25.03.2021 01:00


Mathematics, 25.03.2021 01:00

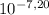 , thus,
, thus, 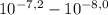 = 5,31x10⁻⁸ M
= 5,31x10⁻⁸ M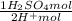 = 8,96x10⁻³ moles of H₂SO₄
= 8,96x10⁻³ moles of H₂SO₄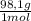 ×
× 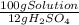 ×
× 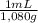 =
= 

