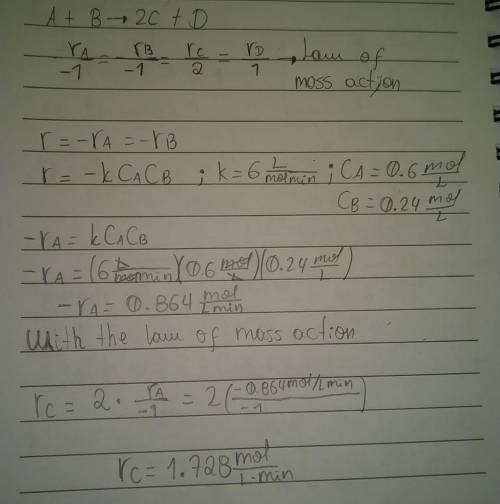Chemistry, 24.09.2019 20:00 soulspiritsa
What is the instantaneous rate of formation of product c given the following information: a. stoichiometric equation a+ b2c+ d b. applicable rate equation is r.-k"ca"cb c. the rate constant is 6.0 liters/(mole-minute) d. the current concentrations of a and b species are ca 0.6 moles/liter and ca 0.24 moles/liter

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2

Chemistry, 22.06.2019 10:30
Earth's axis of rotation is tilted at an angle of 23.5 degrees. what is one change you would see on earth if its axis was not tilted?
Answers: 3

Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3
You know the right answer?
What is the instantaneous rate of formation of product c given the following information: a. stoich...
Questions

Advanced Placement (AP), 23.01.2020 11:31

Mathematics, 23.01.2020 11:31



Biology, 23.01.2020 11:31

Mathematics, 23.01.2020 11:31

English, 23.01.2020 11:31

Biology, 23.01.2020 11:31

Mathematics, 23.01.2020 11:31


English, 23.01.2020 11:31

History, 23.01.2020 11:31

History, 23.01.2020 11:31

Chemistry, 23.01.2020 11:31


Social Studies, 23.01.2020 11:31


Biology, 23.01.2020 11:31

English, 23.01.2020 11:31