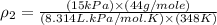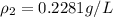Chemistry, 24.09.2019 19:00 saskiat1155
10 m3 of carbon dioxide is originally at a temperature of 50 °c and pressure of 10 kpa. determine the new density and volume of the carbon dioxide if the temperature and pressure change to 75 oc and 15 kpa.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1


Chemistry, 23.06.2019 15:30
Consider these four line graphs representing speed in meters/second, where each x-axis is labelled in seconds and each y-axis is labelled in meters. which line graph indicates the greatest speed at 5 seconds?
Answers: 1
You know the right answer?
10 m3 of carbon dioxide is originally at a temperature of 50 °c and pressure of 10 kpa. determine th...
Questions


Mathematics, 07.02.2021 20:20

Mathematics, 07.02.2021 20:20

Mathematics, 07.02.2021 20:20

Social Studies, 07.02.2021 20:20



English, 07.02.2021 20:20












Mathematics, 07.02.2021 20:20

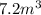 respectively.
respectively.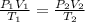
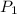 = initial pressure of gas = 10 kPa
= initial pressure of gas = 10 kPa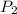 = final pressure of gas = 15 kPa
= final pressure of gas = 15 kPa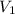 = initial volume of gas =
= initial volume of gas = 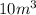
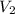 = final volume of gas = ?
= final volume of gas = ?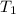 = initial temperature of gas =
= initial temperature of gas = 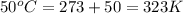
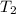 = final temperature of gas =
= final temperature of gas = 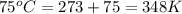
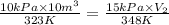
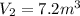
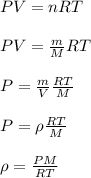
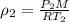
 = new density
= new density