
Chemistry, 24.09.2019 03:20 haydencheramie
The combustion of fuel in your car engine requires oxygen gas, which is supplied as air (21% oxygen molecules) into the engine. consider a car that is using 100% ethanol, c2h5oh, as fuel. if your engine intakes 4.73 l of air per minute at 1.00 atm and 25ºc, what is the maximum volume of ethanol (0.789 g/ml) that can be burned per minute? hint: you can ignore the "per minute" information because both the ethanol and air are being quantified per minute. enter your answer to three significant figures in units of ml.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:50
Which of these reactions are redox reactions? check all that apply.cd + hcl → cdcl2 + h2cucl2 + na2s → 2nacl + cuscaco3 → cao + co2 2zns + 3o2 → 2zno + 2so2 ch4 + 2o2 → co2 + 2h2o
Answers: 3

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1
You know the right answer?
The combustion of fuel in your car engine requires oxygen gas, which is supplied as air (21% oxygen...
Questions



Computers and Technology, 17.06.2020 21:57

English, 17.06.2020 21:57

Mathematics, 17.06.2020 21:57

Mathematics, 17.06.2020 21:57

English, 17.06.2020 21:57



Computers and Technology, 17.06.2020 21:57



Mathematics, 17.06.2020 21:57



Mathematics, 17.06.2020 21:57


Physics, 17.06.2020 21:57

Mathematics, 17.06.2020 21:57

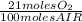 = 0,0406 moles O₂
= 0,0406 moles O₂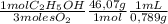 = 0,895 mL of ethanol per minute
= 0,895 mL of ethanol per minute

