Chemistry, 23.09.2019 21:10 sparky1234
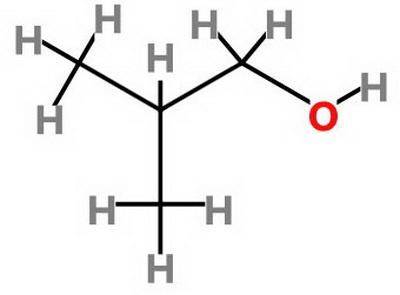
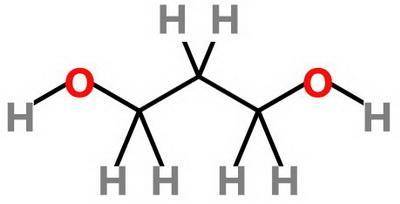
Which statement about 2‑methyl‑1‑propanol, (ch3)2chch2oh , and 1,3‑propanediol, hoch2ch2ch2oh is true? 2‑methyl‑1‑propanol is more soluble in water than 1,3‑propanediol because 2‑methyl‑1‑propanol has a smaller molecular mass. 2‑methyl‑1‑propanol is more soluble in water than 1,3‑propanediol because 2‑methyl‑1‑propanol forms fewer hydrogen bonds with water. 1,3‑propanediol is more soluble in water than 2‑methyl‑1‑propanol because 1,3‑propanediol has a smaller molecular mass. 1,3‑propanediol is more soluble in water than 2‑methyl‑1‑propanol because 1,3‑propanediol can form multiple hydrogen bonds with water.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:30
What’s the scientific notation for the number 6,840,000,000
Answers: 1

Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 21:50
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state.a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2

Chemistry, 22.06.2019 23:00
What is the mass of naoh that would have to be added to 500 ml of a solution of 0.20 m acetic acid in order to achieve a ph of 5.0?
Answers: 1
You know the right answer?
Which statement about 2‑methyl‑1‑propanol, (ch3)2chch2oh , and 1,3‑propanediol, hoch2ch2ch2oh is tru...
Questions












Mathematics, 10.12.2019 04:31