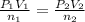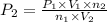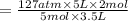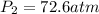Acetylene torches are used for welding. these torches use a mixture of acetylene gas, c2h2, and oxygen gas, o2 to produce the following combustion reaction: 2 c2h2 (g) + 5 o2 (g) → 4 co2 (g) + 2 h2o (g) imagine that you have a 5 l gas tank and a 3.5 l gas tank. you need to fill one tank with oxygen and the other with acetylene to use in conjunction with your welding torch. if you fill the larger tank with oxygen to a pressure of 127 atm , to what pressure should you fill the acetylene tank to ensure that you run out of each gas at the same time? assume ideal behavior for all gases.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1


Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 22.06.2019 17:00
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1
You know the right answer?
Acetylene torches are used for welding. these torches use a mixture of acetylene gas, c2h2, and oxyg...
Questions


Biology, 08.01.2021 16:30



Mathematics, 08.01.2021 16:30

Mathematics, 08.01.2021 16:30

Chemistry, 08.01.2021 16:30

Mathematics, 08.01.2021 16:30

Mathematics, 08.01.2021 16:30


Mathematics, 08.01.2021 16:30


Chemistry, 08.01.2021 16:40






Mathematics, 08.01.2021 16:40

Mathematics, 08.01.2021 16:40

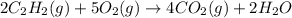
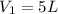
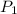 = 127 atm
= 127 atm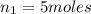
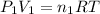
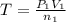 ..[1]
..[1]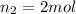
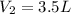
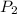 = ?
= ?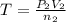 ..[2]
..[2]