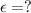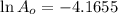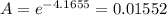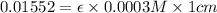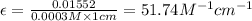Chemistry, 23.09.2019 16:30 peachijmin
Asolution has a concentration of dye= 0.0003 m at a given time during the reaction. the sample described has a ln(a0) = -4.1655. assuming the path length is 1 cm what is the molar absorptivity constant for the dye? report answer to two decimal places. no units required.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 22.06.2019 19:30
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2


Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2
You know the right answer?
Asolution has a concentration of dye= 0.0003 m at a given time during the reaction. the sample descr...
Questions

Social Studies, 08.04.2021 23:00


Business, 08.04.2021 23:00

Biology, 08.04.2021 23:00

English, 08.04.2021 23:00

Mathematics, 08.04.2021 23:00


Computers and Technology, 08.04.2021 23:00





English, 08.04.2021 23:00


Mathematics, 08.04.2021 23:00

Mathematics, 08.04.2021 23:00




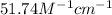 is the molar absorptivity constant for the dye.
is the molar absorptivity constant for the dye.
 = Molar absorptivity constant
= Molar absorptivity constant