
Chemistry, 22.09.2019 05:30 rosehayden21
The n2o4−no2 reversible reaction is found to have the following equilibrium partial pressures at 100∘c. calculate kp for the reaction. n2o4(g)⇌2no2(g) 0.0005 atm 0.095 atm express your answer using two significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2


Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1

You know the right answer?
The n2o4−no2 reversible reaction is found to have the following equilibrium partial pressures at 100...
Questions




History, 22.01.2021 04:20

Biology, 22.01.2021 04:20



Mathematics, 22.01.2021 04:20


Computers and Technology, 22.01.2021 04:20





Social Studies, 22.01.2021 04:20



Mathematics, 22.01.2021 04:20

English, 22.01.2021 04:20

History, 22.01.2021 04:20

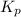 for the reaction is 18.05
for the reaction is 18.05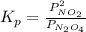
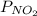 and
and 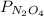 are equilibrium partial pressure of
are equilibrium partial pressure of 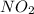 and
and 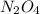 respectively
respectively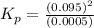 = 18.05
= 18.05


