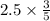Chemistry, 22.09.2019 04:30 sanociahnoel
The combustion of propane (c3h8) in the presence of excess oxygen yields co2 and h20: c3h8 (8) + 5o2 (g) - 3co2 (s) + 4h20 (8) when 2.5 mol of o2 are consumed in their reaction, a) 3.0 b) 7.5 c) 1.5 mol of co2 are produced. d) 4.2 e) 2.5

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. initial mass and yield sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 1

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2

Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3

Chemistry, 23.06.2019 01:00
Which statement characterizes synthetic polymers? a. they come from animals and plants. b. they are found in nature. c. they are made in a lab. d. they are components of starch.
Answers: 1
You know the right answer?
The combustion of propane (c3h8) in the presence of excess oxygen yields co2 and h20: c3h8 (8) + 5o...
Questions

Social Studies, 14.12.2020 21:20

Mathematics, 14.12.2020 21:20

Mathematics, 14.12.2020 21:20



Mathematics, 14.12.2020 21:20

Mathematics, 14.12.2020 21:20

English, 14.12.2020 21:20



Advanced Placement (AP), 14.12.2020 21:20

Arts, 14.12.2020 21:20

Spanish, 14.12.2020 21:20


Mathematics, 14.12.2020 21:20

Mathematics, 14.12.2020 21:20

Mathematics, 14.12.2020 21:20



Mathematics, 14.12.2020 21:20