
Chemistry, 21.09.2019 23:10 ayoismeisalex
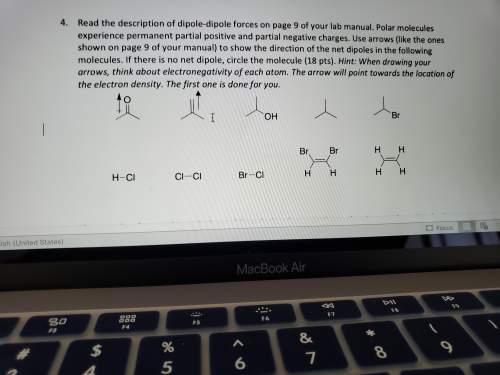
Read the description of dipole-dipole forces on page 9 of your lab manual. polar molecules experience permanent partial positive and partial negative charges. use arrows (like the ones shown on page 9 of your manual) to show the direction of the net dipoles in the following molecules. if there is no net dipole, circle the molecule (18 pts). hint: when drawing your arrows, think about electronegativity of each atom. the arrow will point towards the location of the electron density. the first one is done for you.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
The diagram shows the structures of horse and cat forelimbs. what does the diagram suggest about the evolutionary relationship between these two mammals? a. they have homologous structures, indicating a common ancestor. b. they have analogous structures, indicating a common ancestor. c. they have homologous structures, indicating that they do not have a common ancestor. d. they have analogous structures, indicating that they do not have a common ancestor.
Answers: 2

Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1
You know the right answer?
Read the description of dipole-dipole forces on page 9 of your lab manual. polar molecules experienc...
Questions

Mathematics, 08.03.2021 21:20

Mathematics, 08.03.2021 21:20




Engineering, 08.03.2021 21:20





Mathematics, 08.03.2021 21:20

Social Studies, 08.03.2021 21:20



Mathematics, 08.03.2021 21:20



Mathematics, 08.03.2021 21:20

Mathematics, 08.03.2021 21:20



