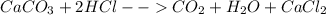Chemistry, 20.09.2019 23:30 sanchezp0821
Tums is a popular remedy for acid indigestion. a typical tums tablet contains calcium carbonate plus some inert substances. when ingested, it reacts with the gastric juice (hydrochloric acid) in the stomach to give off carbon dioxide gas. when a 1.367−g tablet reacted with 40.00 ml of hydrochloric acid (density = 1.140 g/ml), carbon dioxide gas was given off, and the resulting solution weighed 46.699 g. calculate the number of liters of carbon dioxide gas released if its density is 1.81 g/l.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
What volume of a 2.00 m stock solution of naoh is needed to prepare 150. ml of 0.40 m solution?
Answers: 2

Chemistry, 22.06.2019 08:30
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3
You know the right answer?
Tums is a popular remedy for acid indigestion. a typical tums tablet contains calcium carbonate plus...
Questions

Social Studies, 05.05.2020 18:35



Mathematics, 05.05.2020 18:35

Mathematics, 05.05.2020 18:35






Mathematics, 05.05.2020 18:35

Engineering, 05.05.2020 18:35



Mathematics, 05.05.2020 18:35




Mathematics, 05.05.2020 18:35