
Chemistry, 20.09.2019 22:00 destinytofell4630
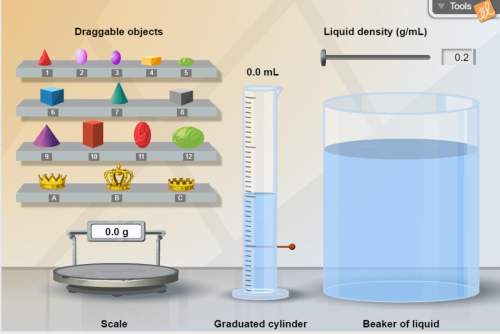
Think about this: how could you devise an easy foolproof way to determine if an object was made of pure gold other than finding the density of the actual object? hint: use the beaker of liquid. briefly explain your method. *

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Noble gases are the most reactive elements on the periodic table. a. true b. false
Answers: 2

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2
You know the right answer?
Think about this: how could you devise an easy foolproof way to determine if an object was made of...
Questions

Mathematics, 02.12.2020 06:30

Mathematics, 02.12.2020 06:30

Mathematics, 02.12.2020 06:30

Physics, 02.12.2020 06:30

Business, 02.12.2020 06:30

English, 02.12.2020 06:30




Social Studies, 02.12.2020 06:30

Mathematics, 02.12.2020 06:30

Mathematics, 02.12.2020 06:30


Social Studies, 02.12.2020 06:30


Mathematics, 02.12.2020 06:30

Mathematics, 02.12.2020 06:30


Computers and Technology, 02.12.2020 06:30

Mathematics, 02.12.2020 06:30



