Chemistry, 20.09.2019 04:00 bryantpropst1395
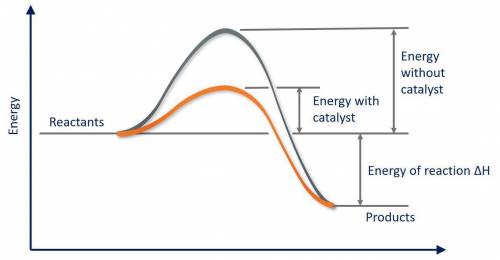
Which of the following statements about catalysts is false? a catalyst provides a different reaction pathway with a lower activation energy. a catalyst cannot affect the overall energy change for the reaction. a catalyst speeds up both the forward and reverse reactions. a catalyst is present before the reaction begins, and is also present in the same form after the reaction ends. a catalyst stabilizes the product of the reaction relative to the reactant.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:00
How many ions that have a +1 charge will bond with an ion that has a -2 charge
Answers: 1

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2
You know the right answer?
Which of the following statements about catalysts is false? a catalyst provides a different reactio...
Questions


Mathematics, 13.11.2020 19:50


Biology, 13.11.2020 19:50


Computers and Technology, 13.11.2020 19:50

Computers and Technology, 13.11.2020 19:50

Mathematics, 13.11.2020 19:50



History, 13.11.2020 19:50

English, 13.11.2020 19:50


Mathematics, 13.11.2020 19:50