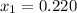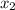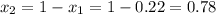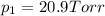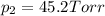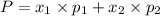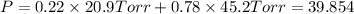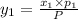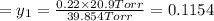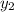Chemistry, 20.09.2019 03:00 aljalloh94
1‑propanol (p∘1=20.9 torr at 25 ∘c) and 2‑propanol (p∘2=45.2 torr at 25 ∘c) form ideal solutions in all proportions. let x1 and x2 represent the mole fractions of 1‑propanol and 2‑propanol in a liquid mixture, respectively, and y1 and y2 represent the mole fractions of each in the vapor phase. for a solution of these liquids with x1=0.220, calculate the composition of the vapor phase at 25 ∘c.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 00:30
Maria wants to determine which type of disinfectant kills the most bacteria. which of the following is the best way for maria to determine this? a. ask ten different companies that make disinfectants which type is best. b. put the same amount and species of bacteria on ten identical plates, and add ten different kinds of disinfectant to each plate. c. interview ten different people to determine which type of disinfectant they prefer. d. put the same amount and species of bacteria on ten identical plates, and add a different disinfectant to each plate.
Answers: 1

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1
You know the right answer?
1‑propanol (p∘1=20.9 torr at 25 ∘c) and 2‑propanol (p∘2=45.2 torr at 25 ∘c) form ideal solutions in...
Questions



Mathematics, 13.08.2020 01:01



English, 13.08.2020 01:01




Mathematics, 13.08.2020 01:01


Mathematics, 13.08.2020 01:01