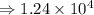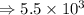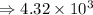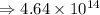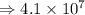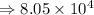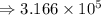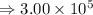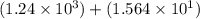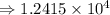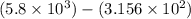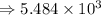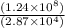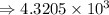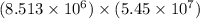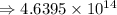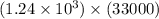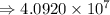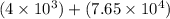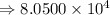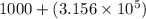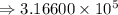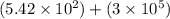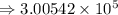Chemistry, 19.09.2019 20:30 ajbrock1004
1) 1.24 x 10^3 + 1.564 x 10^1
2) 5.8 x 10^3 - 3.156 x 10^2
3) 1.24 x 10^8 / 2.87 x 10^4
4) 8.513 x 10^6 x 5.45 x 10^7
5) 1.24 x 10^3 x 33000
6) 4 x 10^3 + 7.65 x 10^4
7) 1000 + 3.156 x 10^5
8) 5.42 x 10^2 + 3 x 10^5

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
City a and city b had two different temperatures on a particular day. on that day, four times the temperature of city a was 8â° c more than 3 times the temperature of city b. the temperature of city a minus twice the temperature of city b was â’3â° c. what was the temperature of city a and city b on that day? city a was 5â° c, and city b was 4â° c. city a was 3â° c, and city b was â’1â° c. city a was 8â° c, and city b was â’3â° c. city a was 5â° c, and city b was â’5â° c.
Answers: 2

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1


Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1
You know the right answer?
1) 1.24 x 10^3 + 1.564 x 10^1
2) 5.8 x 10^3 - 3.156 x 10^2
3) 1.24 x 10^8 / 2.87 x 10^4<...
2) 5.8 x 10^3 - 3.156 x 10^2
3) 1.24 x 10^8 / 2.87 x 10^4<...
Questions

Mathematics, 17.01.2020 00:31

Social Studies, 17.01.2020 00:31

Social Studies, 17.01.2020 00:31

Advanced Placement (AP), 17.01.2020 00:31






Mathematics, 17.01.2020 00:31







Mathematics, 17.01.2020 00:31