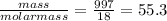Liquids and solids are left out of the equilibrium constant expression because their concentrations remain constant during reactions. what is the molarity concentration of liquid water at 25.0 latex: ^\circ ∘c given that its density is 0.997 g/ml at that temperature?
a.23.5 m
b.0.997 m
c.0.180 m
d.0.156 m
e.55.3 m

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 17:30
What will most likely happen in the absence of a cell membrane? a) photosynthesis will not take place. b) the cell will not store food, water, nutrients, and waste. c) energy will not be released during cellular respiration. d) substances will pass in and out of the cell in an uncontrolled manner.
Answers: 1

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2

You know the right answer?
Liquids and solids are left out of the equilibrium constant expression because their concentrations...
Questions



Mathematics, 21.10.2020 18:01

Mathematics, 21.10.2020 18:01



Social Studies, 21.10.2020 18:01

Arts, 21.10.2020 18:01


English, 21.10.2020 18:01



Mathematics, 21.10.2020 18:01

Computers and Technology, 21.10.2020 18:01



Mathematics, 21.10.2020 18:01


French, 21.10.2020 18:01