
Chemistry, 19.09.2019 20:30 lulabelles7750
Given the following balanced equation at 120°c: a(g) + b(g) ⇋ 2 c(g) + d(s)(a) at equilibrium a 4.0 liter container was found to contain 1.60 moles of a, and 0.40 moles of b, and 0.40 moles of c, and 1.60 moles of d. calculate kc.(b) if 0.20 moles of b and 0.20 mole of c are added to this system, what will be the new equilibrium concentration of a be? (c) if the volume of the container in which the system is at equilibrium [part (a)] is suddenly halved, what will be the new equilibrium concentrations?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 22.06.2019 22:30
What is the work done by the electric force to move a 1 c charge from a to b?
Answers: 2

Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3

Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2
You know the right answer?
Given the following balanced equation at 120°c: a(g) + b(g) ⇋ 2 c(g) + d(s)(a) at equilibrium a 4.0...
Questions


English, 04.10.2021 05:50

Chemistry, 04.10.2021 05:50

Computers and Technology, 04.10.2021 05:50



Mathematics, 04.10.2021 05:50


Social Studies, 04.10.2021 05:50

Chemistry, 04.10.2021 05:50

Chemistry, 04.10.2021 05:50



Business, 04.10.2021 05:50

Mathematics, 04.10.2021 05:50

Computers and Technology, 04.10.2021 05:50


Chemistry, 04.10.2021 05:50

Mathematics, 04.10.2021 05:50

![\frac{[C]^2}{[A][B]}](/tpl/images/0244/0592/0c73c.png)
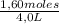 = 0,4 M
= 0,4 M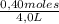 = 0,1 M
= 0,1 M![\frac{[0,1]^2}{[0,4][0,1]}](/tpl/images/0244/0592/6198c.png) = 0,25
= 0,25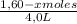
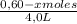
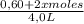
![\frac{[0,60+2x]^2}{[1,60-x][0,60-x]}](/tpl/images/0244/0592/06e92.png)
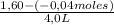 = 0,41 M
= 0,41 M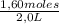 = 0,8 M
= 0,8 M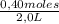 = 0,2 M
= 0,2 M


