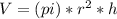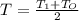An ideal gas in a cylindrical container of radius r and height h is kept at constant pressure p. the bottom of the container is maintained at temperature t0 while the top at temperature t1. assuming a linear temperature distribution along the cylinder, calculate the total mass of gas within the container.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3
You know the right answer?
An ideal gas in a cylindrical container of radius r and height h is kept at constant pressure p. the...
Questions

History, 13.11.2020 22:00


Arts, 13.11.2020 22:00

History, 13.11.2020 22:00


Mathematics, 13.11.2020 22:00

Mathematics, 13.11.2020 22:00


Mathematics, 13.11.2020 22:00





Advanced Placement (AP), 13.11.2020 22:00


Biology, 13.11.2020 22:00

Advanced Placement (AP), 13.11.2020 22:00

Spanish, 13.11.2020 22:00

Mathematics, 13.11.2020 22:00

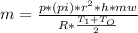
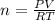
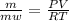 or
or 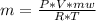 (equation 2)
(equation 2)