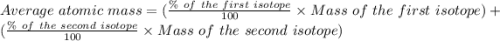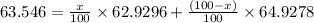Chemistry, 19.09.2019 16:30 xxaurorabluexx
Copper has two naturally occurring isotopes, 63cu (isotopic mass 62.9296 amu) and 65cu (isotopic mass 64.9278 amu). if copper has an atomic mass of 63.546 amu, what is the percent abundance of each isotope? report your answer to 5 significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3


Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

You know the right answer?
Copper has two naturally occurring isotopes, 63cu (isotopic mass 62.9296 amu) and 65cu (isotopic mas...
Questions

Health, 11.01.2020 17:31

Mathematics, 11.01.2020 17:31

History, 11.01.2020 17:31

Mathematics, 11.01.2020 17:31


English, 11.01.2020 17:31

Physics, 11.01.2020 17:31

Mathematics, 11.01.2020 17:31


Spanish, 11.01.2020 17:31

Mathematics, 11.01.2020 17:31


History, 11.01.2020 17:31

World Languages, 11.01.2020 17:31

Business, 11.01.2020 17:31

Mathematics, 11.01.2020 17:31


Chemistry, 11.01.2020 17:31

History, 11.01.2020 17:31

History, 11.01.2020 17:31