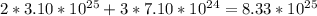Chemistry, 19.09.2019 05:30 kevinh2683
Asolution of ammonia and water contains 3.10×1025 water molecules and 7.10×1024 ammonia molecules. how many total hydrogen atoms are in this solution?

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 00:30
Nuclear decay is the spontaneous decay of one element into a. an x-ray b. a ray of light c. another element
Answers: 1

Chemistry, 23.06.2019 01:00
Which substance—wood or silver—is the better thermal conductor? a thermal conductor is a material that requires very little heat energy to change its temperature. explain your answer.
Answers: 3

Chemistry, 23.06.2019 02:30
Calculate the ph at the equivalence point for the titration of a solution containing 150.0 mg of ethylamine (c2h5nh2) with 0.1000 m hcl solution. the volume of the solution at the equivalence point is 250.0 ml. kb forethylamine is 4.7 × 10−4 .
Answers: 2

Chemistry, 23.06.2019 04:40
6) (a) calculate the absorbance of the solution if its concentration is 0.0278 m and its molar extinction coefficient is 35.9 l/(mol cm). the depth of the cell is 5 mm. (b) what is the %t? (7) calculate the absorbance of the solution if the transmitted light intensity is 70% of the initial light beam intensity
Answers: 1
You know the right answer?
Asolution of ammonia and water contains 3.10×1025 water molecules and 7.10×1024 ammonia molecules. h...
Questions

Mathematics, 31.07.2019 00:00




Mathematics, 31.07.2019 00:00

Physics, 31.07.2019 00:00

Social Studies, 31.07.2019 00:00







Mathematics, 31.07.2019 00:00







 molecules
molecules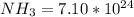 molecules
molecules