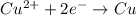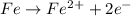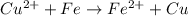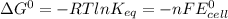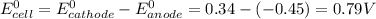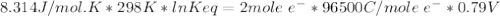Chemistry, 19.09.2019 04:20 IkarosSakurai
Agalvanic cell consists of a iron electrode in 1 m fe(no3)2 and a copper electrode in 1 m cu(no3)2. what is the equilibrium constant for this reaction at 25oc?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted into gaseous br atoms. s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 2

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Chemistry, 22.06.2019 21:00
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1

You know the right answer?
Agalvanic cell consists of a iron electrode in 1 m fe(no3)2 and a copper electrode in 1 m cu(no3)2....
Questions

Social Studies, 05.01.2021 23:30

Mathematics, 05.01.2021 23:30




Mathematics, 05.01.2021 23:30

Biology, 05.01.2021 23:30

Mathematics, 05.01.2021 23:30

Mathematics, 05.01.2021 23:30

Mathematics, 05.01.2021 23:30

Mathematics, 05.01.2021 23:30


Social Studies, 05.01.2021 23:30


English, 05.01.2021 23:30

History, 05.01.2021 23:30


Mathematics, 05.01.2021 23:30