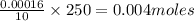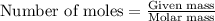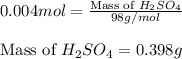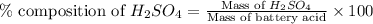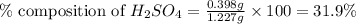Asample of battery acid is to be analyzed for its sulfuric acid content. a 1.00-ml sample weighs 1.227 g . this 1.00-ml sample is diluted to 250.0 ml, and 10.00 ml of this diluted acid requires 35.05 ml of 4.462×10−3 m ba(oh)2 for its titration. what is the mass percent of h2so4 in the battery acid?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2

Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2
You know the right answer?
Asample of battery acid is to be analyzed for its sulfuric acid content. a 1.00-ml sample weighs 1.2...
Questions


Mathematics, 01.04.2020 16:27

Mathematics, 01.04.2020 16:27

Social Studies, 01.04.2020 16:27


English, 01.04.2020 16:27



History, 01.04.2020 16:27


Mathematics, 01.04.2020 16:27







Mathematics, 01.04.2020 16:27

Health, 01.04.2020 16:27

English, 01.04.2020 16:27

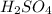 in the battery acid is 31.9 %
in the battery acid is 31.9 %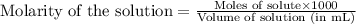
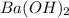 solution =
solution = 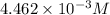
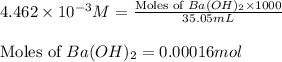
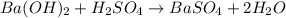
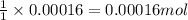 of sulfuric acid.
of sulfuric acid.