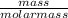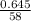Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:30
The length of a vector arrow represents its magnitude and the point represents its direction true or false apex
Answers: 3

Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1
You know the right answer?
Calculate the mass of magnesium oxide produced when 0.645 g of magnesium hydroxide is the is complet...
Questions


Chemistry, 02.11.2020 05:30

Mathematics, 02.11.2020 05:30

Mathematics, 02.11.2020 05:30


Health, 02.11.2020 05:30




Mathematics, 02.11.2020 05:30


Mathematics, 02.11.2020 05:30

Business, 02.11.2020 05:30


Mathematics, 02.11.2020 05:30


English, 02.11.2020 05:30

Social Studies, 02.11.2020 05:30

Biology, 02.11.2020 05:30

Mathematics, 02.11.2020 05:30