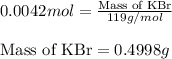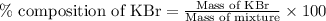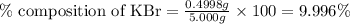Chemistry, 18.09.2019 21:30 Shihhschool20
A5.000 g mixture contains strontium nitrate and potassium bromide. excess lead(ii) nitrate solution is added to precipitate out 0.7822 grams of lead(ii) bromide. what is the percent by mass of potassium bromide in the mixture?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2
You know the right answer?
A5.000 g mixture contains strontium nitrate and potassium bromide. excess lead(ii) nitrate solution...
Questions

Mathematics, 05.11.2020 01:00

Mathematics, 05.11.2020 01:00


Biology, 05.11.2020 01:00

History, 05.11.2020 01:00


Biology, 05.11.2020 01:00

Mathematics, 05.11.2020 01:00

Chemistry, 05.11.2020 01:00



Mathematics, 05.11.2020 01:00

Social Studies, 05.11.2020 01:00






Mathematics, 05.11.2020 01:00

Mathematics, 05.11.2020 01:00

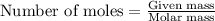 .....(1)
.....(1)
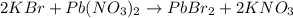
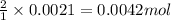 of potassium bromide
of potassium bromide