
Chemistry, 18.09.2019 22:00 camiloriveraveoxbgd6
2mno₄ - (aq) + 5 c₂o₄²⁻ (aq) + 16 h⁺ (aq) → 2 mn²⁺ (aq) + 10 co₂ (g) + 8 h₂o (l)permanganate and oxalate ions react in an acidified solution according to the balanced equation above. how many moles of co₂(g) are produced when 20. ml of acidified 0.20 m kmno₄ solution is added to 50. ml of 0.10 m na₂c₂o₄ solution?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2


Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2

Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2
You know the right answer?
2mno₄ - (aq) + 5 c₂o₄²⁻ (aq) + 16 h⁺ (aq) → 2 mn²⁺ (aq) + 10 co₂ (g) + 8 h₂o (l)permanganate and oxa...
Questions

Chemistry, 25.07.2019 00:00

Biology, 25.07.2019 00:00

Mathematics, 25.07.2019 00:00

History, 25.07.2019 00:00


Biology, 25.07.2019 00:00


Biology, 25.07.2019 00:00


Mathematics, 25.07.2019 00:00

Social Studies, 25.07.2019 00:00




Business, 25.07.2019 00:00




Advanced Placement (AP), 25.07.2019 00:00

Biology, 25.07.2019 00:00

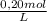 = 4,0×10⁻³ KMnO₄ moles
= 4,0×10⁻³ KMnO₄ moles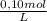 = 5,0×10⁻³ Na₂C₂O₄ moles
= 5,0×10⁻³ Na₂C₂O₄ moles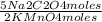 =
=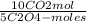 =
= 

