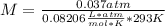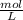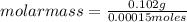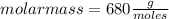Answers: 1


Another question on Chemistry



Chemistry, 23.06.2019 04:10
An unknown substance has been shown to have metallic bonds. which of the following is most likely a property of this substance? a. low conductivity b. low boiling point c. high malleability d. high solubility in water
Answers: 2

You know the right answer?
Asolution containing 0.102 g of an unknown compound dissolved in 100. ml of water has an osmotic pre...
Questions

Mathematics, 05.05.2020 22:58



Geography, 05.05.2020 22:58



Mathematics, 05.05.2020 22:59

History, 05.05.2020 22:59


Physics, 05.05.2020 22:59



English, 05.05.2020 22:59



Spanish, 05.05.2020 22:59

Advanced Placement (AP), 05.05.2020 22:59


Mathematics, 05.05.2020 22:59

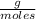
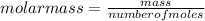
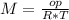
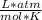 T=20 °C= 293°K (0°C=273 °K)
T=20 °C= 293°K (0°C=273 °K)