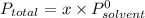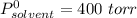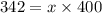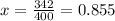Chemistry, 18.09.2019 17:20 lindseyreneesmith7
Diethyl ether has a vapor pressure of 400.0 torr at 18°c. when a sample of benzoic acid (a non-volatile compound) is dissolved in ether, the vapor pressure of the solution is 342 torr. what is the mole fraction of benzoic acid in the solution?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Omg imgonnafailnfiedkla use complete sentences to explain how the mass of hydrogen is conserved during cellular respiration.
Answers: 1

Chemistry, 22.06.2019 01:00
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1
You know the right answer?
Diethyl ether has a vapor pressure of 400.0 torr at 18°c. when a sample of benzoic acid (a non-volat...
Questions




History, 11.03.2020 22:13








English, 11.03.2020 22:14


Social Studies, 11.03.2020 22:14

Mathematics, 11.03.2020 22:14

English, 11.03.2020 22:14

Computers and Technology, 11.03.2020 22:14