Chemistry, 18.09.2019 05:30 asuhdude57
A245.7g sample of metal at 75.0℃ was placed in 115.4g of water at 22.0℃. the final temperature of the water and metal was 34.0℃. if no heat was lost to the surroundings, what is the specific heat of the metal? (specific heat of water = 4.184 j/g℃)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3

Chemistry, 22.06.2019 21:30
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3
You know the right answer?
A245.7g sample of metal at 75.0℃ was placed in 115.4g of water at 22.0℃. the final temperature of th...
Questions

Mathematics, 12.12.2019 10:31

Spanish, 12.12.2019 10:31

Mathematics, 12.12.2019 10:31


Mathematics, 12.12.2019 10:31




Mathematics, 12.12.2019 10:31

Mathematics, 12.12.2019 10:31

History, 12.12.2019 10:31









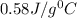
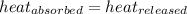
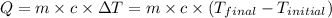
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0238/8184/09236.png) .................(1)
.................(1)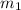 = mass of metal = 245.7 g
= mass of metal = 245.7 g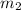 = mass of water = 115.4 g
= mass of water = 115.4 g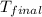 = final temperature =
= final temperature = 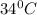
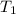 = temperature of metal =
= temperature of metal = 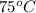
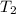 = temperature of water =
= temperature of water = 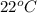
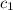 = specific heat of metal = ?
= specific heat of metal = ?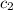 = specific heat of water=
= specific heat of water= 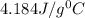
![-245.7\times c\times (75-34)=[115.4\times 4.184\times (34-22)]](/tpl/images/0238/8184/37846.png)