
Chemistry, 18.09.2019 05:20 capybaracaptin2895
A36.0 mg sample of an organic compound (molar mass = 84.0 g/mol) is dissolved in 10.0 ml of water. this aqueous solution is extracted with 5.0 ml of hexane. separation and analysis of the aqueous phase shows that it now contains 12.0 mg of the organic compound. calculate the partition coefficient for the compound between hexane and water (kphexane/water). your answer should reflect the proper number of significant figures.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution? a. 3.88 m, b. 1.03 m, c. 1.5 m, d. 15.5 m
Answers: 3

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2

Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1
You know the right answer?
A36.0 mg sample of an organic compound (molar mass = 84.0 g/mol) is dissolved in 10.0 ml of water. t...
Questions

Mathematics, 24.11.2020 01:40

Mathematics, 24.11.2020 01:40

English, 24.11.2020 01:40


French, 24.11.2020 01:40


Social Studies, 24.11.2020 01:40



Mathematics, 24.11.2020 01:40

Mathematics, 24.11.2020 01:40



Mathematics, 24.11.2020 01:40

Mathematics, 24.11.2020 01:40

English, 24.11.2020 01:40

Physics, 24.11.2020 01:40

Computers and Technology, 24.11.2020 01:40


Mathematics, 24.11.2020 01:40

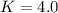
![[solute]_{water}=\frac{12mg*\frac{1mmol}{84.0mg}}{10.0mL}*\frac{1000mL}{1L}=14.3mM](/tpl/images/0238/7804/ef1af.png)
![[solute]_{hexane}=\frac{(36.0-12.0)mg*\frac{1mmol}{84.0mg}}{5mL}*\frac{1000mL}{1L}=57mM](/tpl/images/0238/7804/17da7.png)
![K=\frac{[solute]_{hexane}}{[solute]_{water}} = \frac{57mM}{14.3mM} \\K=4.0](/tpl/images/0238/7804/d9577.png)


