
Chemistry, 18.09.2019 05:00 jngonzo1226
Amixture of oxygen and hydrogen is analyzed by passing it over hot copper oxide and through a drying tube. hydrogen reduces the cuo to metallic cu. oxygen then reoxidizes the copper back to cuo. we know that 100 cm3 of the mixture measured at 25o c and 750 mm yields 84.5 cm3 of dry oxygen measured at at 25o c and 750mm after passage over cuo and the drying agent. what is the original composition of the mixture? (hint: first write balanced chemical equation for the reactions.)

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 06:00
In an exothermic reaction at equilibrium, what is the effect of lowering the temperature? a. the reaction makes more products. b. the reaction makes more reactants. c. the reaction is unchanged.
Answers: 1

Chemistry, 23.06.2019 06:30
Which of these natural resources is non-renewable a.corn b.wind c.geothermal d.natural gas
Answers: 2


Chemistry, 23.06.2019 11:20
Match each state of matter with the statement that best describes it.
Answers: 1
You know the right answer?
Amixture of oxygen and hydrogen is analyzed by passing it over hot copper oxide and through a drying...
Questions


Mathematics, 06.05.2020 02:25

History, 06.05.2020 02:26

History, 06.05.2020 02:26

Mathematics, 06.05.2020 02:26

Mathematics, 06.05.2020 02:26


History, 06.05.2020 02:26






Mathematics, 06.05.2020 02:26

History, 06.05.2020 02:26

Mathematics, 06.05.2020 02:26

Mathematics, 06.05.2020 02:26


Mathematics, 06.05.2020 02:26

Mathematics, 06.05.2020 02:26

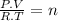
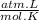
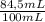 = 3,41x10⁻³ moles O₂
= 3,41x10⁻³ moles O₂ 

