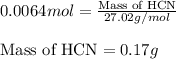Chemistry, 18.09.2019 04:10 potaetoo1997
When potassium cyanide (kcn) reacts with acids, a deadly poisonous gas, hydrogen cyanide (hcn), is given off: kcn(aq) + hcl(aq) → hcn(g) + kcl(aq) if a sample of 0.420 g of kcn is treated with an excess of hcl, calculate the amount of hcn formed in grams.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2

Chemistry, 22.06.2019 18:30
How many moles of lead are in 1.50 x 10^12 atoms of lead? could you explain the answer as well and not just give it to me i am refreshing for finals and i need to know how to do it
Answers: 3

Chemistry, 22.06.2019 23:00
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1

Chemistry, 23.06.2019 01:00
Which of the following is the molecular formula for a simple sugar? a. cooh b. h2o c. oh d. c6h12o6
Answers: 1
You know the right answer?
When potassium cyanide (kcn) reacts with acids, a deadly poisonous gas, hydrogen cyanide (hcn), is g...
Questions

English, 03.07.2019 01:00

History, 03.07.2019 01:00


Mathematics, 03.07.2019 01:00

Mathematics, 03.07.2019 01:00




Spanish, 03.07.2019 01:00




History, 03.07.2019 01:00

Mathematics, 03.07.2019 01:00


Mathematics, 03.07.2019 01:00




Spanish, 03.07.2019 01:00

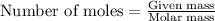 .....(1)
.....(1)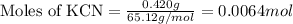
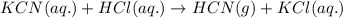
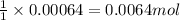 of hydrogen cyanide
of hydrogen cyanide