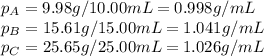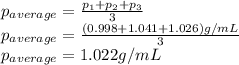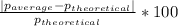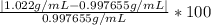Chemistry, 18.09.2019 04:10 christianskyy7074
Density measurements were conducted on a 22.5oc sample of water which had a theoretical density of 0.997655 g/ml. a volume of 10.00 ml of the water had a mass of 9.98 g. a volume of 15.00 ml of the water had a mass of 15.61 g. a volume of 25.00 ml of the water had a mass of 25.65 g. (1) show the calculation of the density of each volume. (2) show the calculation of the average density. (3) show the calculation of the percent error based on the theoretical density of 0.997655 g/ml.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 04:30
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2
You know the right answer?
Density measurements were conducted on a 22.5oc sample of water which had a theoretical density of 0...
Questions



Mathematics, 15.04.2021 04:40

Mathematics, 15.04.2021 04:40


Mathematics, 15.04.2021 04:40

Mathematics, 15.04.2021 04:40


Mathematics, 15.04.2021 04:40

Mathematics, 15.04.2021 04:40


Mathematics, 15.04.2021 04:40

Chemistry, 15.04.2021 04:40

Mathematics, 15.04.2021 04:40

Social Studies, 15.04.2021 04:40



Physics, 15.04.2021 04:40

Mathematics, 15.04.2021 04:40