Chemistry, 18.09.2019 03:30 Ruthsybel9754
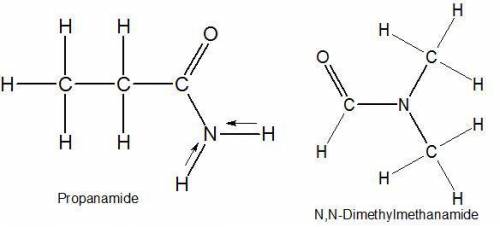
Select the single best answer. why is the boiling point of propanamide, ch3ch2conh2, considerably higher than the boiling point of n, n−dimethylformamide, hcon(ch3)2 (213°c vs. 153°c), even though both compounds are isomeric amides? ch3ch2conh2 has weaker intermolecular forces than hcon(ch3)2 hydrogen bonding is present in ch3ch2conh2 but not in hcon(ch3)2 ch3ch2conh2 is more branched than hcon(ch3)2 ch3ch2conh2 has two methyl groups

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which other elements contain the same number of outer electrons as sodium
Answers: 3

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2


You know the right answer?
Select the single best answer. why is the boiling point of propanamide, ch3ch2conh2, considerably hi...
Questions


Mathematics, 28.03.2020 22:39

Mathematics, 28.03.2020 22:39


Mathematics, 28.03.2020 22:39

Mathematics, 28.03.2020 22:39

English, 28.03.2020 22:39


Mathematics, 28.03.2020 22:39

Mathematics, 28.03.2020 22:39

Computers and Technology, 28.03.2020 22:39

Mathematics, 28.03.2020 22:39

Mathematics, 28.03.2020 22:39

Mathematics, 28.03.2020 22:39

Chemistry, 28.03.2020 22:39



History, 28.03.2020 22:39