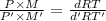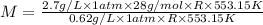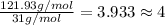Chemistry, 18.09.2019 02:00 davisparker5269
At its boiling point (280°c) and at atmospheric pressure, phosphorus gas has a density of 2.7 g l–1 . under the same conditions, nitrogen gas has a density of 0.62 g l–1 . how many atoms of phosphorus are there in one phosphorus molecule under these conditions?

Answers: 3


Another question on Chemistry




Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2
You know the right answer?
At its boiling point (280°c) and at atmospheric pressure, phosphorus gas has a density of 2.7 g l–1...
Questions




Mathematics, 03.08.2021 06:10

Business, 03.08.2021 06:10

Health, 03.08.2021 06:10

Social Studies, 03.08.2021 06:10



Social Studies, 03.08.2021 06:10

Mathematics, 03.08.2021 06:10


Mathematics, 03.08.2021 06:20



Mathematics, 03.08.2021 06:20

Mathematics, 03.08.2021 06:20

English, 03.08.2021 06:20

Mathematics, 03.08.2021 06:20

Mathematics, 03.08.2021 06:20

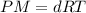
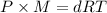 ...(1)
...(1)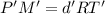
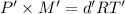 ...(2)
...(2)