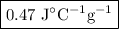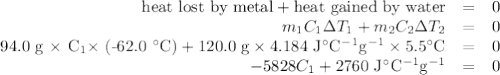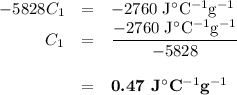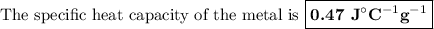A74.0-gram piece of metal at 94.0 °c is placed in 120.0 g of water in a calorimeter at 26.5 °c. the final temperature in the calorimeter is 32.0 °c. determine the specific heat of the metal. show your work by listing various steps, and explain how the law of conservation of energy applies to this situation.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2


Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3

Chemistry, 23.06.2019 05:00
Asolution is made by dissolving 2.3 moles of sodium chloride (nacl) in 0.155 kilograms of water. if the molal boiling point constant for water (kb) is 0.51 °c/m, what would be the boiling point of this solution? show all the steps taken to solve this problem.
Answers: 1
You know the right answer?
A74.0-gram piece of metal at 94.0 °c is placed in 120.0 g of water in a calorimeter at 26.5 °c. the...
Questions







Mathematics, 02.06.2020 00:58





Mathematics, 02.06.2020 00:58

Computers and Technology, 02.06.2020 00:58

Computers and Technology, 02.06.2020 00:58

Mathematics, 02.06.2020 00:58



Biology, 02.06.2020 00:58

Mathematics, 02.06.2020 00:58

Mathematics, 02.06.2020 00:58